Robotic Radiation Therapy
Client
Empyrean
Practice Areas
Challenge
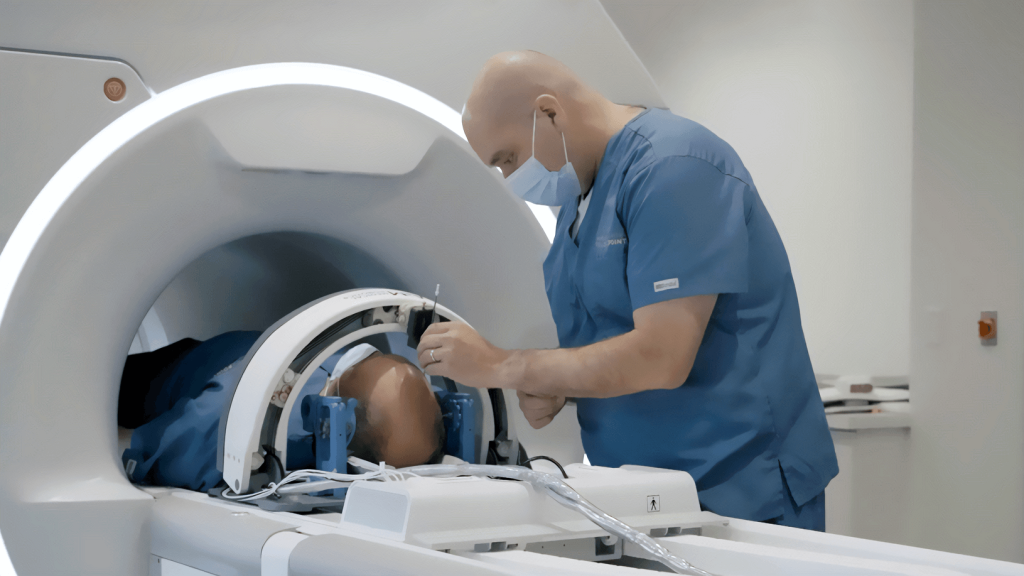
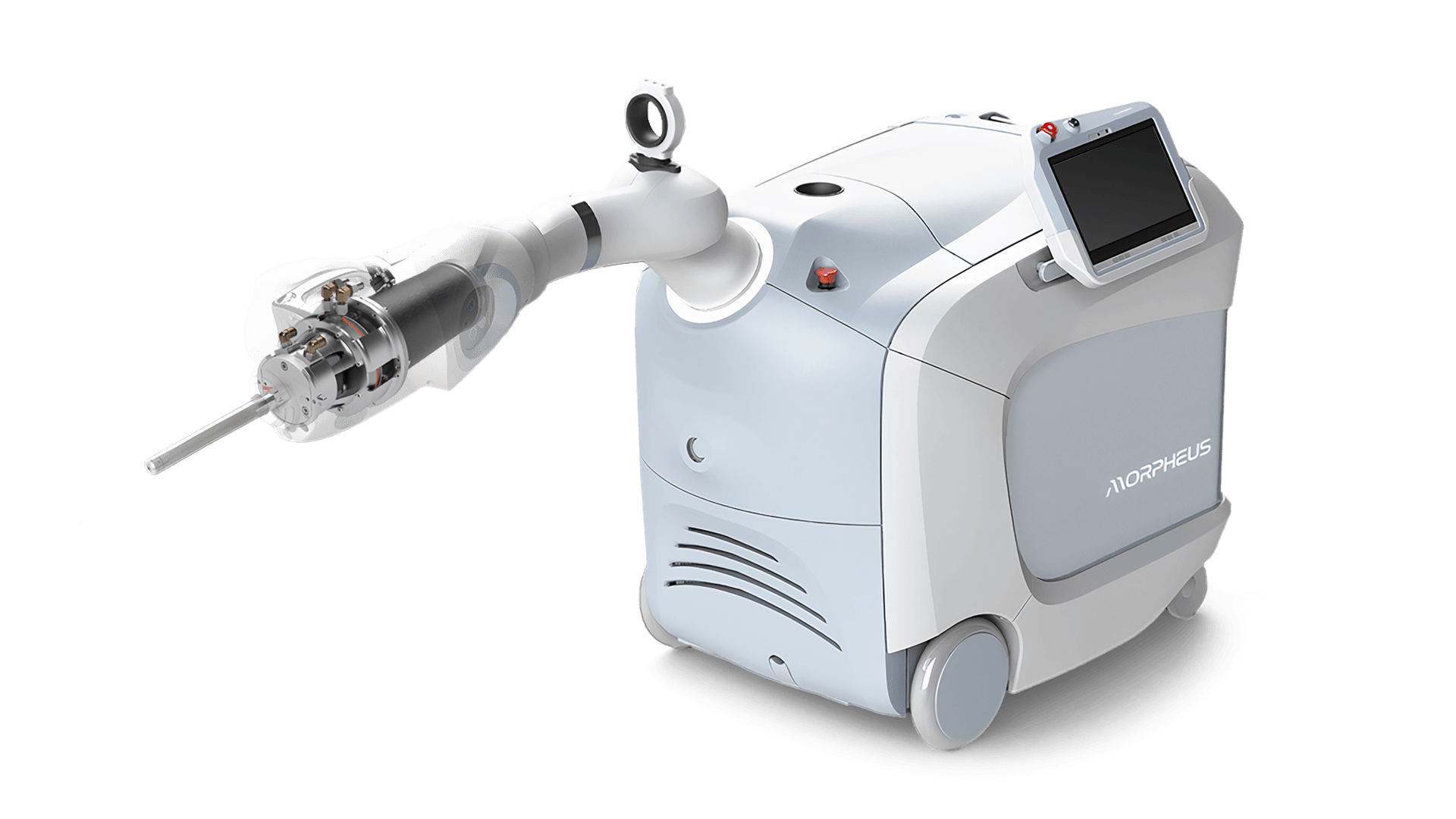
Triple Ring Technologies designed and developed a novel intra-operative radiation therapy device for Empyrean Medical Systems and facilitated submission of a complete design package to FDA for 510(k) clearance. The compact, mobile, robotically guided, low-energy, intra-operative radiation therapy device was designed with the patient in mind and with a tight focus on usability.
Outcomes
A full system was designed, integrated, verified, clinically validated, and submitted to FDA for marketing clearance. Post 510(k), the device design was transferred to manufacture and launched into the market.
Triple Ring Value Proposition
- Innovative custom x-ray source design for true 3D beam directionality
- Valuable intellectual property created
- Advanced modeling and Monte Carlo simulation to speed design process
- System integration and transfer to manufacture services
- Strong track record of FDA clearance for device designs
Robotic Radiation Therapy
Challenge
Triple Ring Technologies designed and developed a novel intra-operative radiation therapy device for Empyrean Medical Systems and facilitated submission of a complete design package to FDA for 510(k) clearance. The compact, mobile, robotically guided, low-energy, intra-operative radiation therapy device was designed with the patient in mind and with a tight focus on usability.
Outcomes
A full system was designed, integrated, verified, clinically validated, and submitted to FDA for marketing clearance. Post 510(k), the device design was transferred to manufacture and launched into the market.
Triple Ring Value Proposition
- Innovative custom x-ray source design for true 3D beam directionality
- Valuable intellectual property created
- Advanced modeling and Monte Carlo simulation to speed design process
- System integration and transfer to manufacture services
- Strong track record of FDA clearance for device designs
Client
Empyrean
Practice Areas


Background
Empryean Medical and Triple Ring collaborated on the Morpheus System throughout the product development life cycle from concept to market launch. Triple Ring’s contributions included design and development of critical subsystems (x-ray source and beam steering electronics, for examples) to system integration, verification and test, clinical validation, and FDA submission. Triple Ring was chosen as Empyrean’s co-development partner due to our vast experience commercializing complex energy delivery technology for a range of clinical applications.