MedTech Strategist’s global investment & networking conference: Innovation Summit Dublin 2020
Note that this event has been rescheduled. The new dates are September 7 – 9 , 2020 at The Shelbourne Hotel, Dublin, Ireland
To our Clients and Partners:
As we all continue to adapt to the evolving situation around the coronavirus, we would like to express our sincere hope that you and your families are safe and sound, and we’d like to give you an update regarding how Triple Ring is responding to the outbreak.
Like many companies, Triple Ring’s staff is now working almost entirely from their home offices or, in some cases, their garages. Given the nature of our business, our team is well-equipped to operate in a distributed fashion and, as a result, we have been able to continue focusing on our clients’ work with minimal interruption. The only change you are likely to notice is more home offices in the background on video calls, as well as the occasional dog barking during a meeting. Other than that, we are 100% open for business.
We are proud to say that many of our team members are working on projects that are directly related to COVID-19. In some cases those team members need to be on site and working in our labs. When this is necessary, we are taking every precaution to keep our staff safe and are closely following or exceeding CDC guidelines.
We encourage you to stay in touch and let us know how we can help you. You have our commitment that we will continue to do whatever we can to support our clients, partners, and employees during this situation and beyond.
We will all get through this. Together.
Thank you.
Joseph A Heanue, Ph.D.
President & CEO
The March 5th, 2020 event has been canceled due to the Covid-19 outbreak in the Bay Area.
Triple Ring’s Senior Engineer Xinyi Zhou, PhD will present a talk on Wednesday Mar 4 at 12:10 pm in the Point of Care Diagnostics Track
Title: Reducing Manufacture Costs of Autologous Cellular Immunotherapy via a Benchtop System for QA/QC Automation
Abstract: Autologous cell therapies have shown unprecedented promise for patients with previously incurable disease. However, skyrocketing costs limit patient access to these life-saving therapies. QA/QC of autologous cell therapies accounts for up to 50% of manufacture cost due to the need for QA/QC of a small (single-patient) batch. We present a prototype for benchtop automation of common sterility and purity assays. A compact QC/QA instrument paired with disposable cartridges can reduce labor costs in cell therapy manufacture and increase patient access to care.
You can achieve a dual vision: improve the impact on people’s health, and build liquidity for your company. The road to successful medical device technologies that win on both fronts is possible—with some key considerations.
Gaining traction requires clinical and regulatory competency, no doubt. But what’s not on your radar may be the source of delays and pitfalls, and is often what keeps companies from hitting milestones. Having to reconfigure your product or re-conduct a single trial can be the expense (both dollars and time) breaks an emerging company. Many elements can be effectively leveraged in the device development process to increase chances of success:
— Early validation of market desirability
— Human factors & usability
— Manufacturing preparation
Early engagement and integration of these activities has a definite payoff. New technologies can be brought to market expediently, without sacrificing safety or effectiveness.
Panelists include:
SHANNON CLARK, CEO at UserWise
NOREEN KING, President & CEO at Evolve Manufacturing
HOWARD EDELMAN, Chairman Medical Devices & Digital Health Screening Committee, Life Science Angels; Entrepreneur-in-Residence at UCSF
Moderator:
DOUG HIEMSTRA, Practice Area Lead – Medical Devices, Triple Ring Technologies
Triple Ring will be at the Emerging MedTech Summit in Dana Point
Roger Tang’s talk is on Wed, Feb 12th at 4:05 PM in room 210B: The Two Mindsets of Successful MD Manufacturing. This is a joint presentation with Aaron Joseph of Sunstone Pilot.
Walt Cecka’s talk is on Thursday Feb 13th at 2:45 PM in room 210D: Lightning Workshop: Congratulations, You’ve Received Your 510(k). So, Is Your Product Really Successful?. This is a joint presentation with Siddharth Desai)
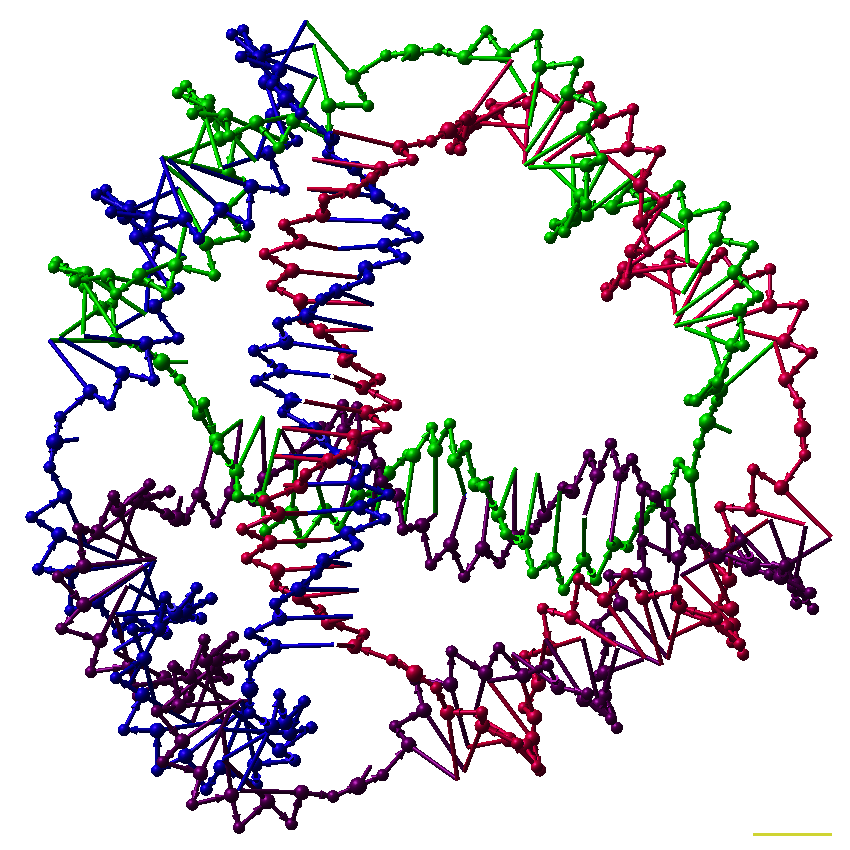
Supramolecular biomaterials exploit rationally-designed non-covalent interactions to enable innovative approaches to drug formulation and delivery. For example, supramolecular interactions can be used to dynamically cross-link polymer networks, yielding shear-thinning and self-healing hydrogels that allow for minimally invasive implantation in vivo though direct injection or catheter delivery to tissues. Alternatively, rationally designed high-affinity interactions can be used to non-covalently modify therapeutic proteins, endowing them with prosthetic function such as enhanced stability in formulation or extended activity in vivo. In this talk, we will discuss the investigation of a hydrogel platform exploiting dynamic multivalent interactions between biopolymers and nanoparticles. These materials exhibit viscous flow under shear stress (shear-thinning) and rapid recovery of mechanical properties when the applied stress is relaxed (self-healing), afford minimally invasive implantation in vivo though direct injection. The hierarchical construction of these biphasic hydrogels allows for multiple therapeutic compounds to be entrapped simultaneously and delivered with identical release profiles, regardless of their chemical make-up, over user-defined timeframes ranging from days to months. These materials enable novel approaches to immunomodulatory therapies such as vaccines and cancer immunotherapies that rely on precise and sustained release of complex mixtures of compounds. We demonstrate that these unique characteristics enable the development of vaccines that greatly enhance the magnitude, quality, and durability of the humoral immune response.
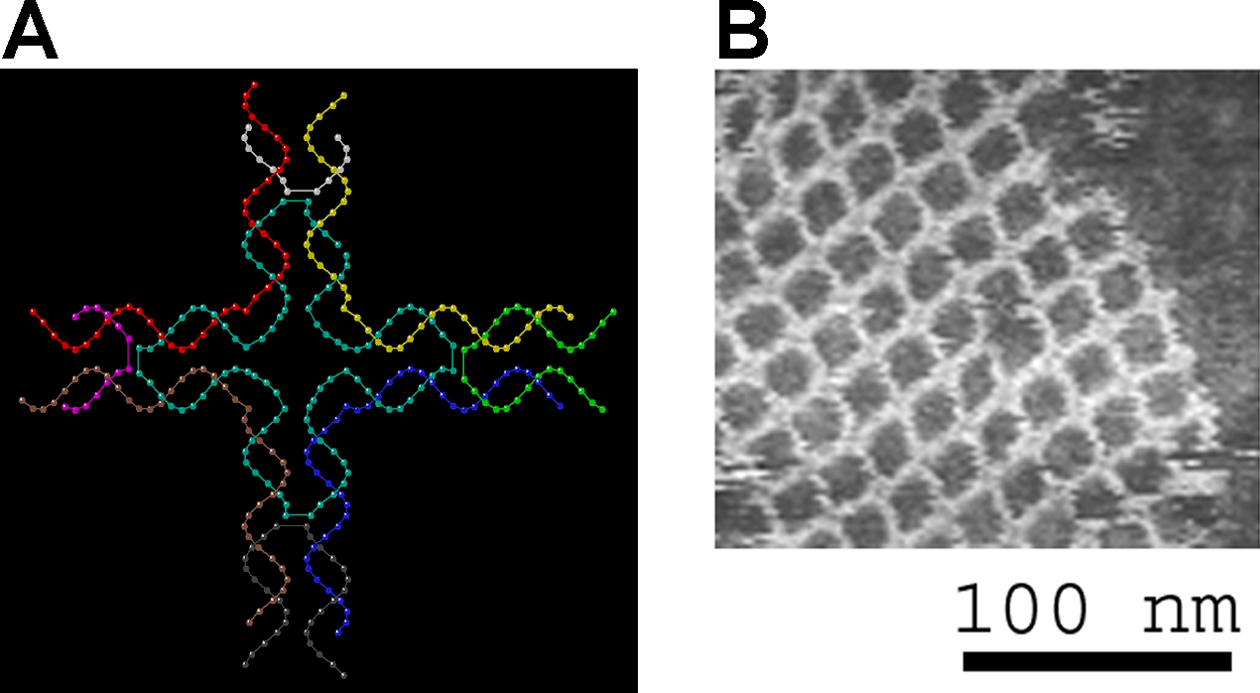
Further, we will discuss the use of supramolecular interactions to append functionality to therapeutic proteins to enhance their stability in formulation and therapeutic function. This non-covalent approach to modification of authentic proteins is highly modular and allows for formulation of historically incompatible proteins. Overall, this presentation will demonstrate the utility of a supramolecular approach to the design of biomaterials affording unique opportunities in the formulation and controlled release of therapeutics.
Images are licensed under Creative Commons License.
Triple Ring will be at Photonics West with our partner, Ocean Insight. We will be at BiOS Hall D, booth #8202 on Sat & Sun and then at Photonics West Hall B, booth #1127 on Tue, Wed, and Thurs.