We’re looking forward to hearing the latest in the world of personalized medicine
1/14 Join us at RESI
We’ll be navigating the JP Morgan crowds this week in San Francisco. For a full listing of events, consult the always helpful MacDougall list
Triple Ring is attending CES 2020. We’re excited to see the latest advances in consumer health, mobility, and communication.
On December 12th and 13th, Triple Ring will be in San Francisco to attend the VRX conference, featuring a wide range of virtual-reality applications.
Meet the Triple Ring team and our partners from Evolve Manufacturing at BIOMEDevice in San Jose.
Join us for MedTech Frontiers. Jim Fruchterman, a leading social entrepreneur, will present Technology: Doing Good on Purpose, Rather than Evil (By Accident?)

Technology has immense power to help people. A single-minded focus on profits often requires companies to ignore 95% of humanity for excellent business reasons. Sometimes, companies mistake their economic interest as a valid reason to do things that actively hurt human beings. Our speaker will provide a more optimistic vision of how technologists, entrepreneurs and investors can choose a more positive path, using the innovation tools of Silicon Valley to maximize human benefit while still honoring most of the constraints of our modern capitalist system. He will share his path from helping blow up a private enterprise rocket, building venture-backed machine learning companies in Silicon Valley, and how he found his life’s work adapting the latest tech innovations to serve the other 95% of humanity.
Images are licensed under Creative Commons License.
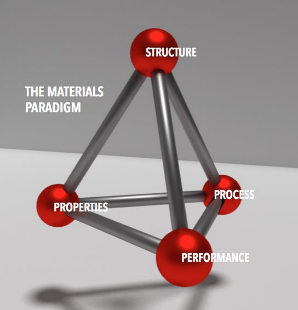
While the development of medical and pharmaceutical devices not only takes time and money, it is also highly regulated. Selecting the right choice of material and process is essential in order to meet compliance regulations, ensure certification processes and reproducibility, and most importantly, provide a high quality product.
Dr. Bhatt will discuss several medical grade polymer materials which have been developed, exhibiting unique characteristics that offer extraordinary benefits over non-polymeric materials in the modern healthcare system. Additionally, these materials comply with global regulatory requirements that ensure they maintain their integrity without causing secondary and/or long-term complications in the patient. Using a specific case study, “Material Development for a Hip Implant Application”, he will discuss the development process and requirements to wade through the myriad of stages In addition, the following 5 criteria must be evaluated thoroughly: (1) product design and application specifications; (2) material properties and consideration; (3) cost; (4) regulatory and handling aspects; and (5) manufacturing and quality plan(s).
Images are licensed under Creative Commons License.

The concept of Habitable Zone (HZ) was once the only way to estimate the chances of a planet to support life. The past decades of exploration in the Solar System and the study of terrestrial extreme environments have shown that the subsurface and interior of several planets and moons located outside the HZ had – or may still have – conditions suitable for the development and survival of life. Beyond the Solar System, the discovery of thousands of exoplanets gives us a chance to expand our understanding of planetary system formation and evolution, and infer their ability to develop biology. These recent discoveries also give us important information about the probability for the existence of other technologically advanced civilizations in the universe. While the current estimates of life potential, whether simple or complex, are based on concepts such as planetary habitability and the coevolution of life and environment, emerging new theories bridge biology, neuroscience, information technology, and quantum physics, and if verified, would fundamentally change our views on the origin and nature of life, and the meaning of its exploration.
Images are licensed under Creative Commons License.