
Flow Cytometer Data Analysis
Client
Beckman Coulter Life Sciences
Practice Areas
Challenge
The CytoFLEX platform from Beckman Coulter Life Sciences is a market-leading flow cytometry platform powered by a unique collection of innovative technologies. The CytoFLEX platform offers versatility, exceptional detection capabilities, low maintenance, automated sample processing, and a comprehensive software subsystem for device control and data analysis. In an effort to gain market advantage over competing third party data analysis products, Beckman Coulter Life Sciences initiated a project with Triple Ring that demanded multiple disciplines collaborating closely and moving quickly. Deploying experts in optics, algorithm development, data science, and software development Triple Ring created algorithms which enable Beckman Coulter Life Sciences to flexibly address multiple high-value customer use cases in the future.
Value Propositions
Triple Ring blended deep optics know-how with advanced algorithm and software engineering to turn Beckman Coulter’s vision for next-generation CytoFLEX analytics into reality.
- Proven experience developing robust constrained, ill-posed optimization algorithms for real-world data
- Rapid creation of high-value IP and products that expand market share and support long-term growth
- Future proofing of legacy hardware through addition of advanced software and algorithms
- Flexible software development capabilities to support varying levels of stringency, from engineering tools to regulated medical device software
- Rapid ramp up to R&D productivity through deep analysis, technical know-how, and Seamless integration with client’s technical team
Flow Cytometer Data Analysis
Challenge
The CytoFLEX platform from Beckman Coulter Life Sciences is a market-leading flow cytometry platform powered by a unique collection of innovative technologies. The CytoFLEX platform offers versatility, exceptional detection capabilities, low maintenance, automated sample processing, and a comprehensive software subsystem for device control and data analysis. In an effort to gain market advantage over competing third party data analysis products, Beckman Coulter Life Sciences initiated a project with Triple Ring that demanded multiple disciplines collaborating closely and moving quickly. Deploying experts in optics, algorithm development, data science, and software development Triple Ring created algorithms which enable Beckman Coulter Life Sciences to flexibly address multiple high-value customer use cases in the future.
Value Propositions
Triple Ring blended deep optics know-how with advanced algorithm and software engineering to turn Beckman Coulter’s vision for next-generation CytoFLEX analytics into reality.
- Proven experience developing robust constrained, ill-posed optimization algorithms for real-world data
- Rapid creation of high-value IP and products that expand market share and support long-term growth
- Future proofing of legacy hardware through addition of advanced software and algorithms
- Flexible software development capabilities to support varying levels of stringency, from engineering tools to regulated medical device software
- Rapid ramp up to R&D productivity through deep analysis, technical know-how, and Seamless integration with client’s technical team
Client
Beckman Coulter Life Sciences
Practice Areas

Background
A key to the longstanding market success of the CytoFLEX platform from Beckman Coulter Life Sciences is a sophisticated data analysis software suite that allows users to predict unknown properties of their test samples. At project initiation, Triple Ring was tasked with designing algorithms to expand the number of high-value customer use cases from two to ten. Beckman Coulter Life Sciences expects that the new use cases will enable the capture of market value held by third-party solutions. Beckman Coulter Life Sciences chose to work with Triple Ring due to our versatile expertise in optics and algorithm development, as well as our proven success on similar projects.
Engagement with Triple Ring has been very positive. The team was very receptive, understanding and dedicated! I was very pleased with the support we received at all levels of the organization
Hani El-Sheikh, Global Head of R&D – Flow Cytometry at Beckman Coulter Life Sciences
The Triple Ring team greatly appreciated working alongside the world-class R&D organization at Beckman Coulter Life Sciences. It was especially gratifying to augment their capabilities with our hard-to-find mix of optics, algorithm development, data science, and software engineering expertise.
Daniel Badali, Ph.D.
More information on the Beckman Coulter Life Sciences website

Cannabis Breathalyzer System
Client
Hound Labs
Practice Areas
Challenge
Hound Labs partnered with Triple Ring Technologies to invent a point-of-use breath analyzer to meet the needs of law enforcement agencies across the world and employers in states where recreational marijuana use is legal. Starting from a napkin sketch, Triple Ring, alongside Hound Labs, created a high-sensitivity portable breathalyzer system tailored to law enforcement and employment settings. The project included ground-breaking validation, and peer reviewed publication, of ∆-9 THC pharmacodynamics in exhaled breath.
Outcomes
Fully functioning prototype systems were created featuring an easy-to-use breath capture device, a control station, and low-cost microfluidic lab-on-a-chip cartridges. Clinical-grade data were robustly produced at the point-of-need to provide indications of recent marijuana use and possible impairment.
Triple Ring Value Proposition
- Full R&D teams, on demand, flexibly resourced from concept to validation
- Outsourced Tough Tech product development expertise
- Deep expertise in biological sciences and bioengineering
- Extensive intellectual property creation owned outright by client
- Financial stake and shared-risk engagement models
- Access to Agility Labs incubation facilities
Cannabis Breathalyzer System
Challenge
Hound Labs partnered with Triple Ring Technologies to invent a point-of-use breath analyzer to meet the needs of law enforcement agencies across the world and employers in states where recreational marijuana use is legal. Starting from a napkin sketch, Triple Ring, alongside Hound Labs, created a high-sensitivity portable breathalyzer system tailored to law enforcement and employment settings. The project included ground-breaking validation, and peer reviewed publication, of ∆-9 THC pharmacodynamics in exhaled breath.
Outcomes
Fully functioning prototype systems were created featuring an easy-to-use breath capture device, a control station, and low-cost microfluidic lab-on-a-chip cartridges. Clinical-grade data were robustly produced at the point-of-need to provide indications of recent marijuana use and possible impairment.
Triple Ring Value Proposition
- Full R&D teams, on demand, flexibly resourced from concept to validation
- Outsourced Tough Tech product development expertise
- Deep expertise in biological sciences and bioengineering
- Extensive intellectual property creation owned outright by client
- Financial stake and shared-risk engagement models
- Access to Agility Labs incubation facilities
Client
Hound Labs
Practice Areas
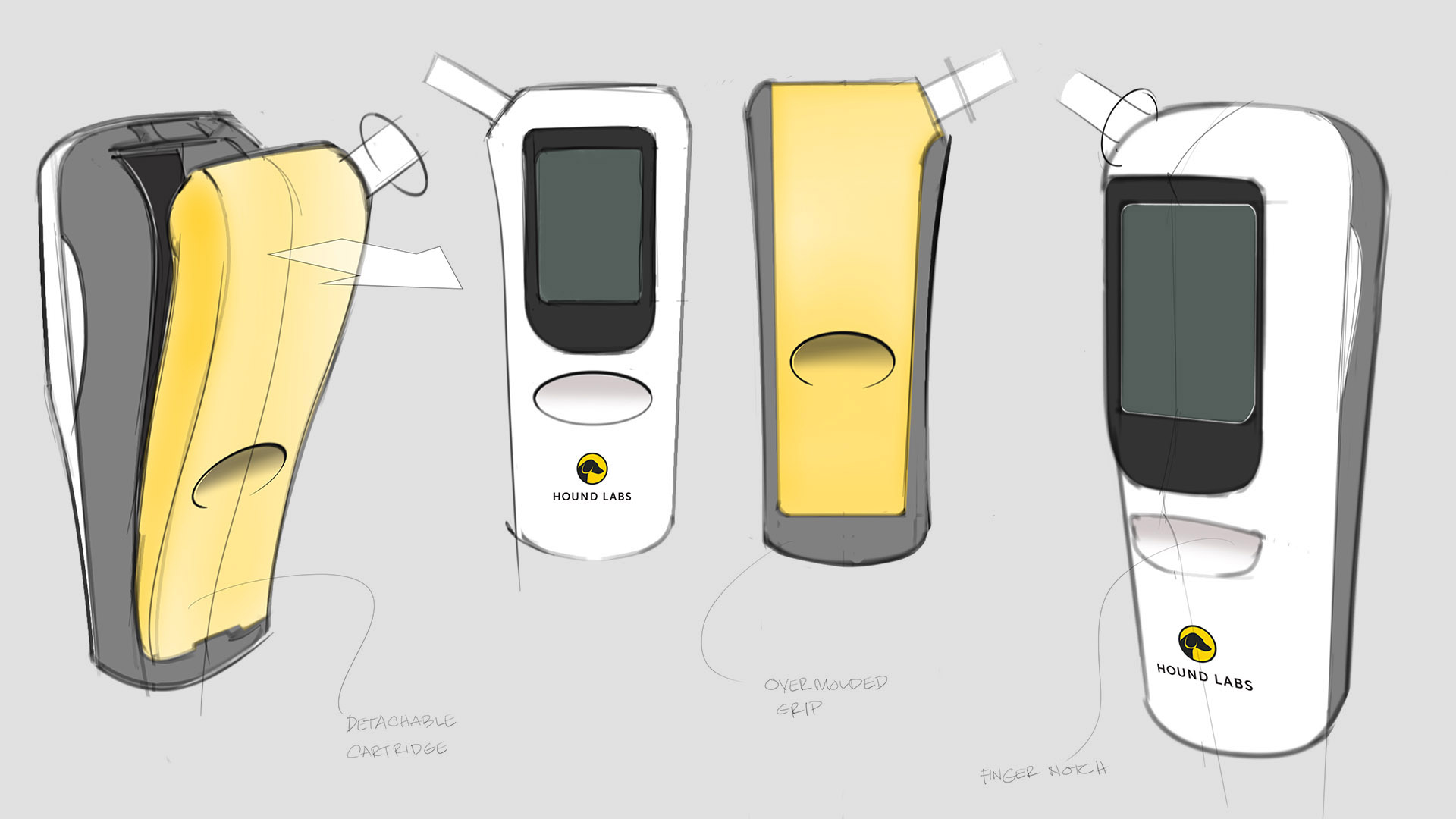
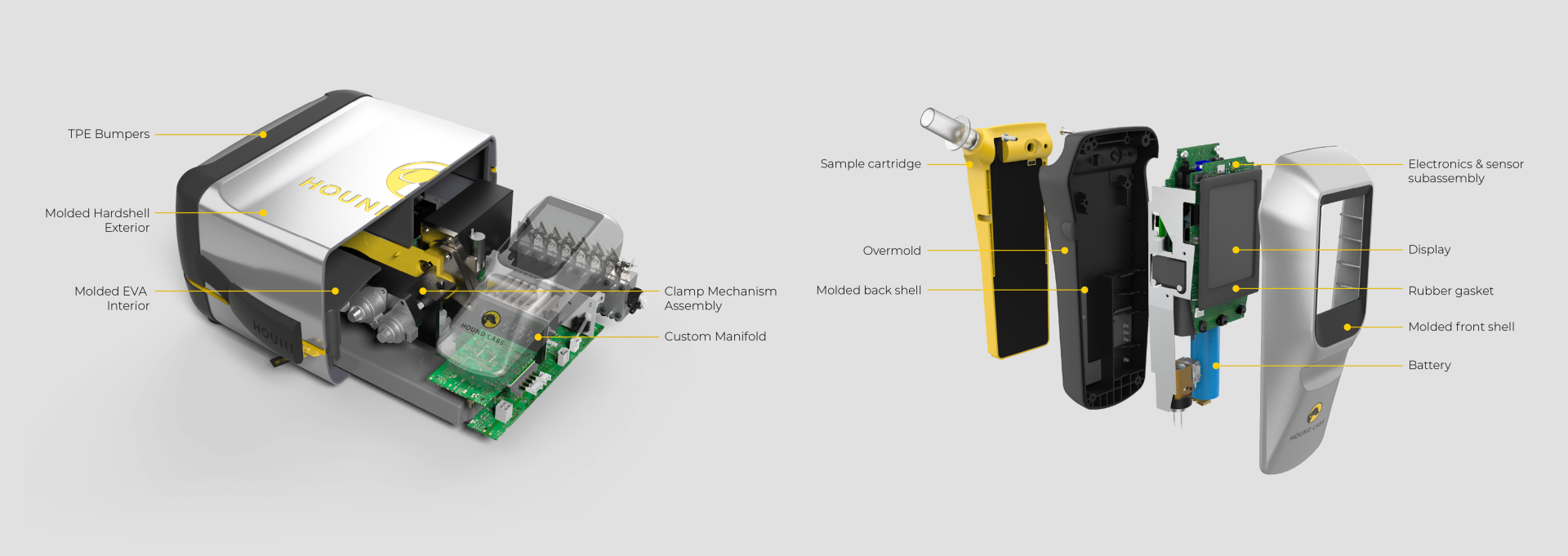
Background
Hound Labs markets a first-in-class system for breath-based assessment of recent marijuana use. The company founders chose Triple Ring as their product development partner for our deep life sciences expertise and for our reputation of helping innovators do the seemingly impossible. In a collaboration that has lasted years, Triple Ring has been a trusted partner demonstrating high degrees of urgency, commitment, and ownership as this cutting-edge startup built products that satisfy critical market needs.


Transdermal GFR Measurement
Client
MediBeacon
Practice Areas
Challenge
MediBeacon required expert assistance to develop a photonics-based transdermal detection technology to monitor intravenously injected fluorescent tracer agents and provide clinically actionable measures of kidney function. MediBeacon has brought combination products into the clinic which match fluorescent tracers with innovative detection systems to provide accurate physiological measurements in real-time. Triple Ring, in collaboration with MediBeacon, designed and delivered a non-invasive, light-based wearable detection system as a critical component of their glomerular filtration rate (GFR) monitoring product. The detectors were designed and developed to meet strict product requirements of sensitivity, accuracy, cost, comfort, and ease of use.
Outcomes
Fully integrated wearable GFR detectors were developed under ISO13485 design controls and delivered for critical clinical trials.
Triple Ring Value Proposition
- Highly relevant expertise in light-based tissue analysis
- Advanced modeling and simulations to speed design process
- Program structure that included skin in the game
- Deep experience in meeting critical milestones for venture funding
Transdermal GFR Measurement
Challenge
MediBeacon required expert assistance to develop a photonics-based transdermal detection technology to monitor intravenously injected fluorescent tracer agents and provide clinically actionable measures of kidney function. MediBeacon has brought combination products into the clinic which match fluorescent tracers with innovative detection systems to provide accurate physiological measurements in real-time. Triple Ring, in collaboration with MediBeacon, designed and delivered a non-invasive, light-based wearable detection system as a critical component of their glomerular filtration rate (GFR) monitoring product. The detectors were designed and developed to meet strict product requirements of sensitivity, accuracy, cost, comfort, and ease of use.
Outcomes
Fully integrated wearable GFR detectors were developed under ISO13485 design controls and delivered for critical clinical trials.
Triple Ring Value Proposition
- Highly relevant expertise in light-based tissue analysis
- Advanced modeling and simulations to speed design process
- Program structure that included skin in the game
- Deep experience in meeting critical milestones for venture funding
Client
MediBeacon
Practice Areas
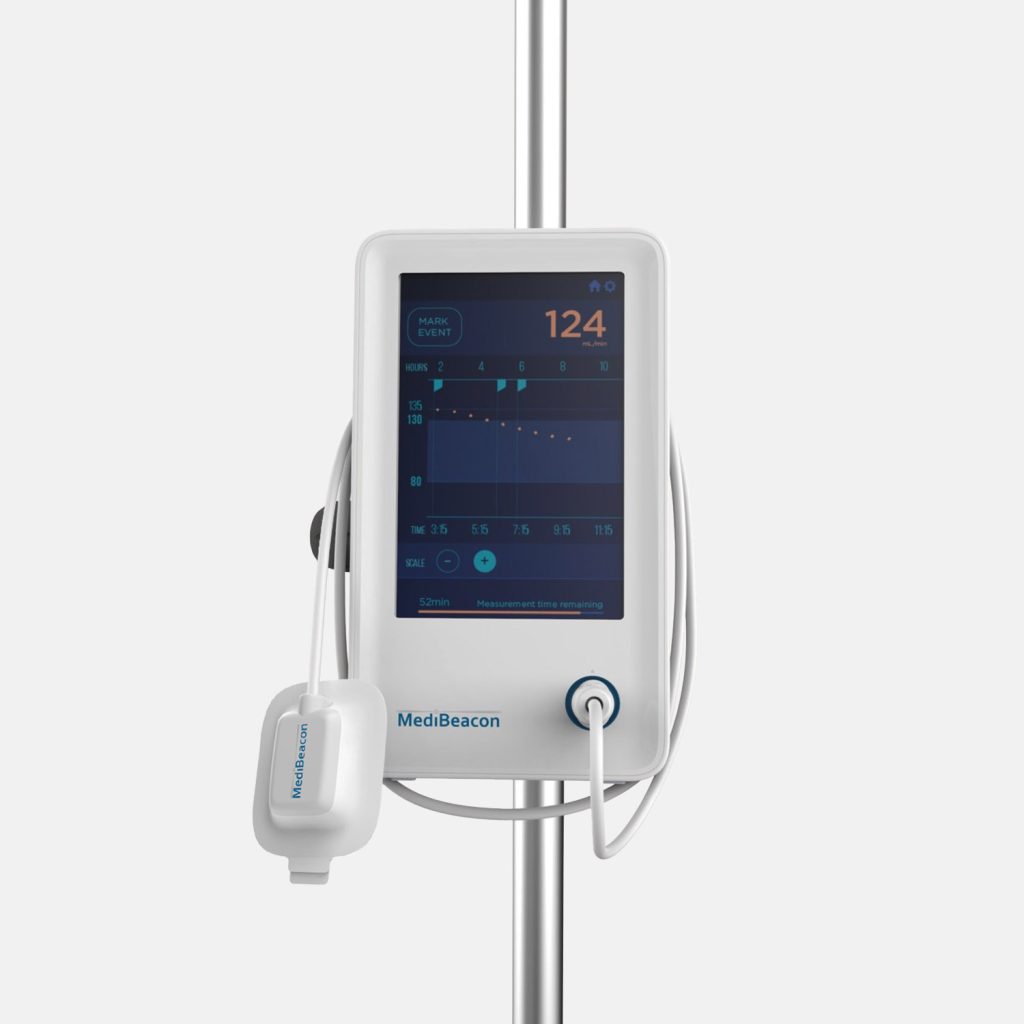
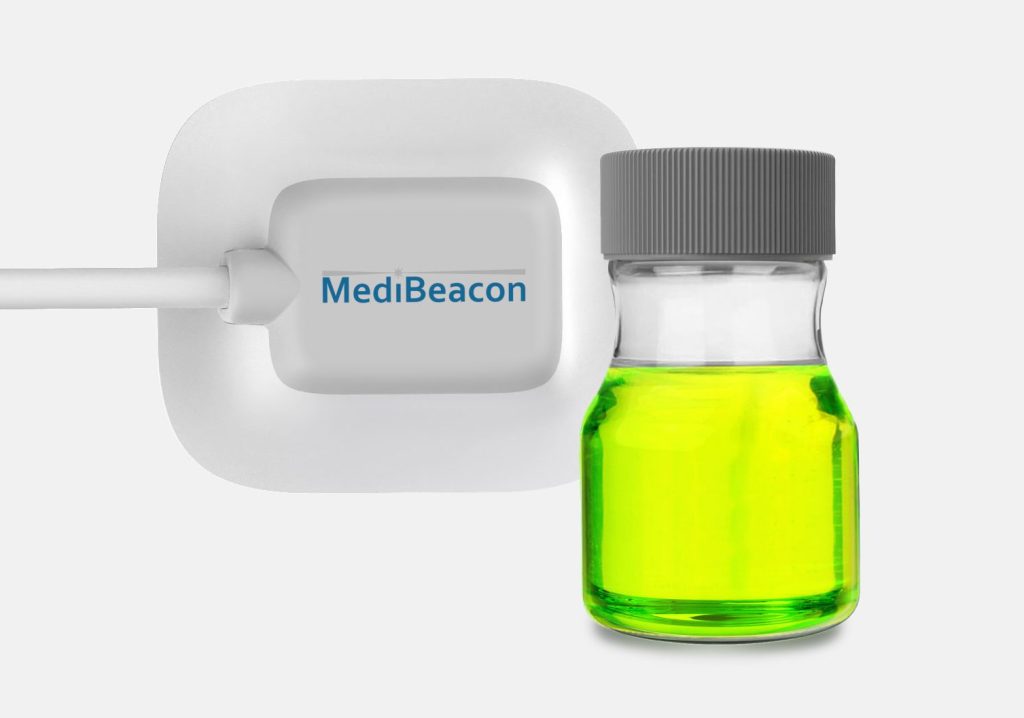
Background
MediBeacon selected Triple Ring as their development partner for our multidisciplinary science and engineering teams. Triple Ring’s industry leading capabilities in biophotonics and combination product development, along with our ability to collaborate closely and share risk, made us an optimal partner for MediBeacon.


Ocean Microplastics Monitor
Client
Various
Practice Areas
Challenge
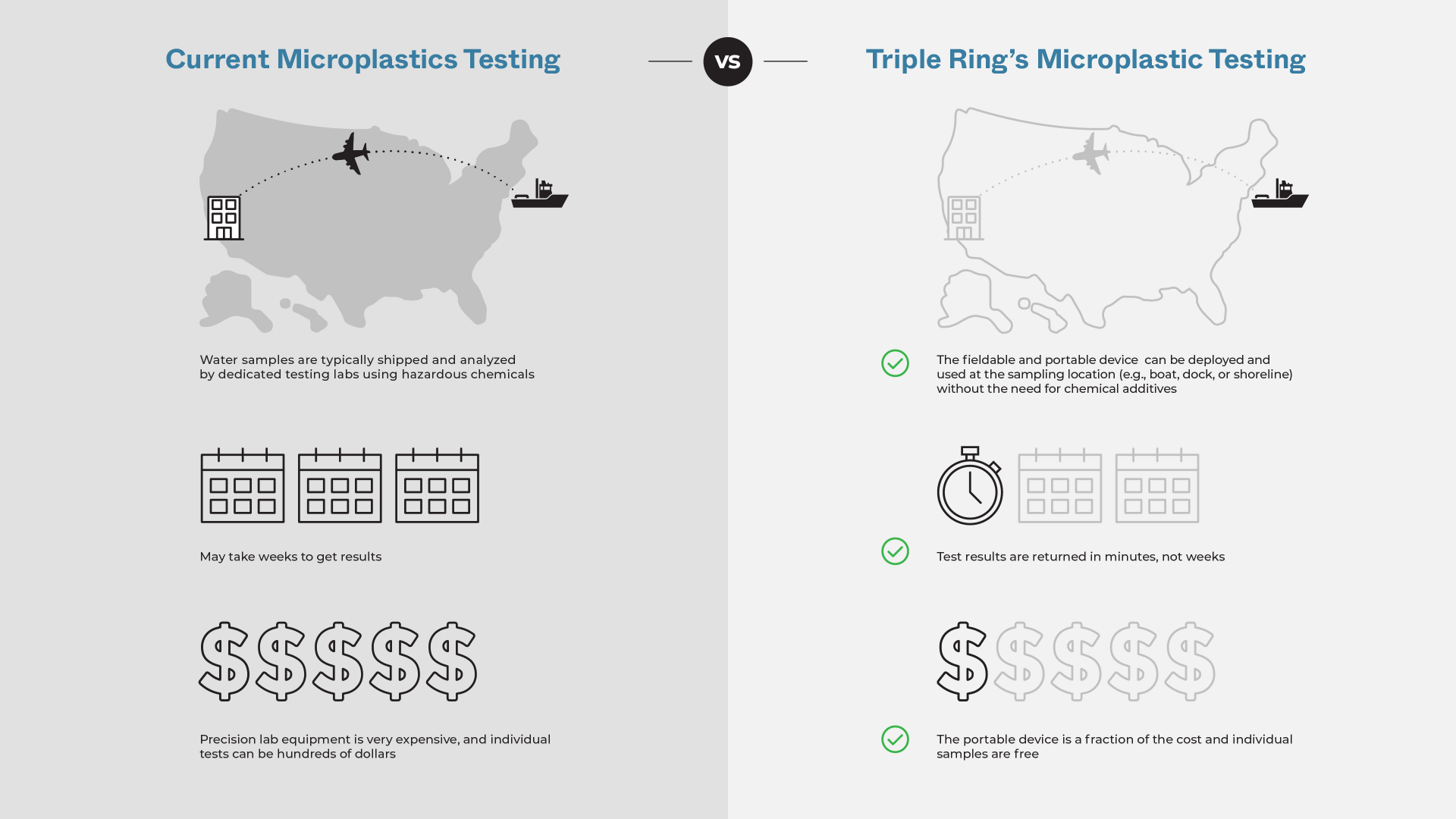
Addressing a critical unmet need in environmental sensing, Triple Ring Technologies supported the integration of a fieldable, portable microplastics sensor system. The system enables quantitation of plastic particles in aqueous environmental samples and separates plastics from non-plastics for subsequent polymer identification. Triple Ring experts created an integrated and miniaturized unit that replaced a high-end instrument in a benchtop system. This unit was designed to successfully count microplastics under conditions expected in field use – not only measuring plastic but also working correctly in the presence of interferents, such as air bubbles and non-plastics (biological particles, sand, silt, and other non-organic materials).
Outcomes
The resulting system could be deployed beyond the benchtop and provided successful demonstrations to funders and partners as the research group furthers its work on microplastics.
Value Propositions
- Relevant expertise in low-cost, ruggedized life sciences assay platforms
- Rapid design and development process that met strict timelines for delivery
- Flexible and highly collaborative design teams that interface seamlessly with research institutes
Ocean Microplastics Monitor
Challenge
Addressing a critical unmet need in environmental sensing, Triple Ring Technologies supported the integration of a fieldable, portable microplastics sensor system. The system enables quantitation of plastic particles in aqueous environmental samples and separates plastics from non-plastics for subsequent polymer identification. Triple Ring experts created an integrated and miniaturized unit that replaced a high-end instrument in a benchtop system. This unit was designed to successfully count microplastics under conditions expected in field use – not only measuring plastic but also working correctly in the presence of interferents, such as air bubbles and non-plastics (biological particles, sand, silt, and other non-organic materials).
Outcomes
The resulting system could be deployed beyond the benchtop and provided successful demonstrations to funders and partners as the research group furthers its work on microplastics.
Value Propositions
- Relevant expertise in low-cost, ruggedized life sciences assay platforms
- Rapid design and development process that met strict timelines for delivery
- Flexible and highly collaborative design teams that interface seamlessly with research institutes
Client
Various
Practice Areas
Background
The US Environmental Protection Agency (EPA) saw value in funding the Triple Ring and Woods Hole Oceanographic Institution teams to produce a fieldable microplastics monitoring system. Triple Ring personnel brought previous experience of shipboard microplastic measurement from past collaborations with EPA in the Northwest Hawaiian Islands. Woods Hole scientists invented a novel means of measuring ocean microplastics and Triple Ring provided integration support.
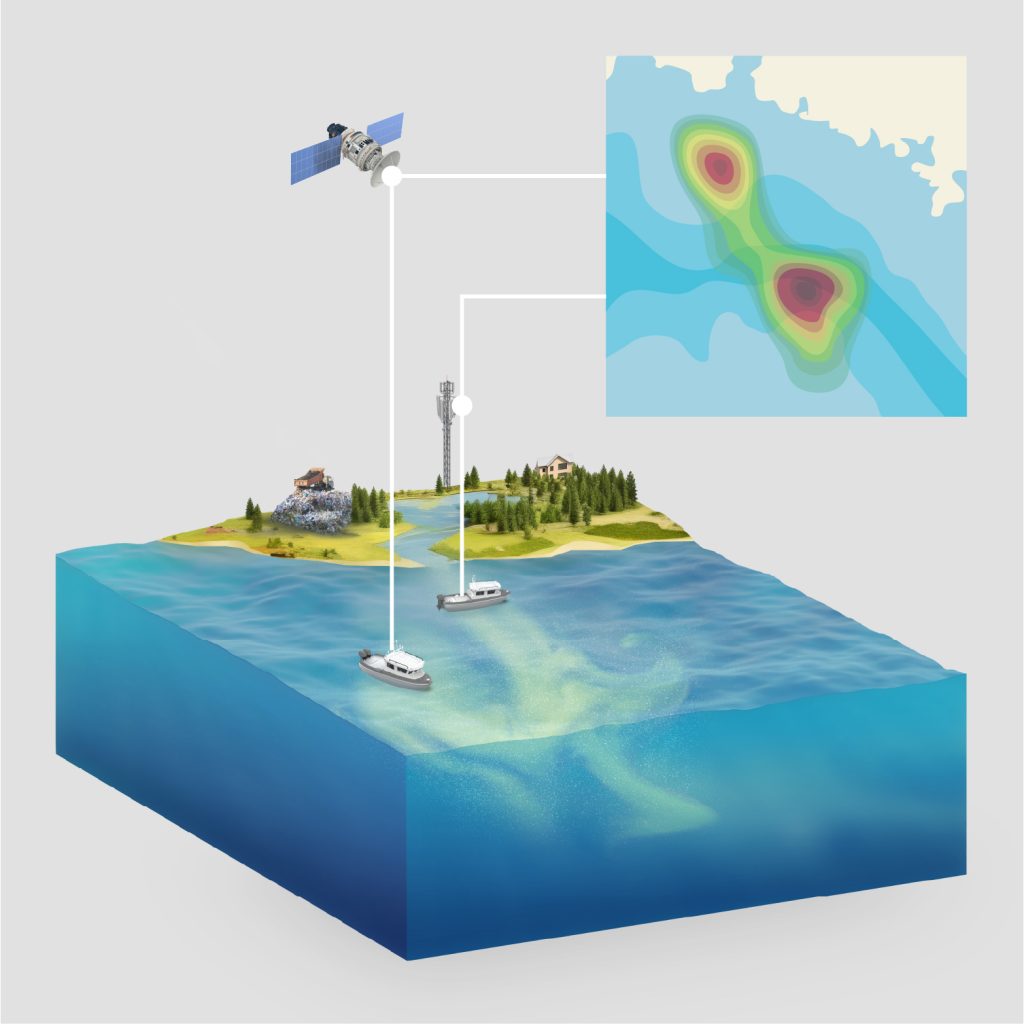

Combining WHOI’s core sensing technology with Triple Ring’s experience in delivering integrated products will significantly move the needle on the understanding of microplastic pollution and will drive data-based mitigation solutions.
My lab is especially interested in developing technologies that enable us to robustly count the number of microplastics in drinking water, ponds, lakes, and oceans. We need field-deployable sensors that provide us this information in order for us to understand microplastic pollution.

At-Home Quantitative Diagnostics
Client
BARDA
Practice Areas
Challenge
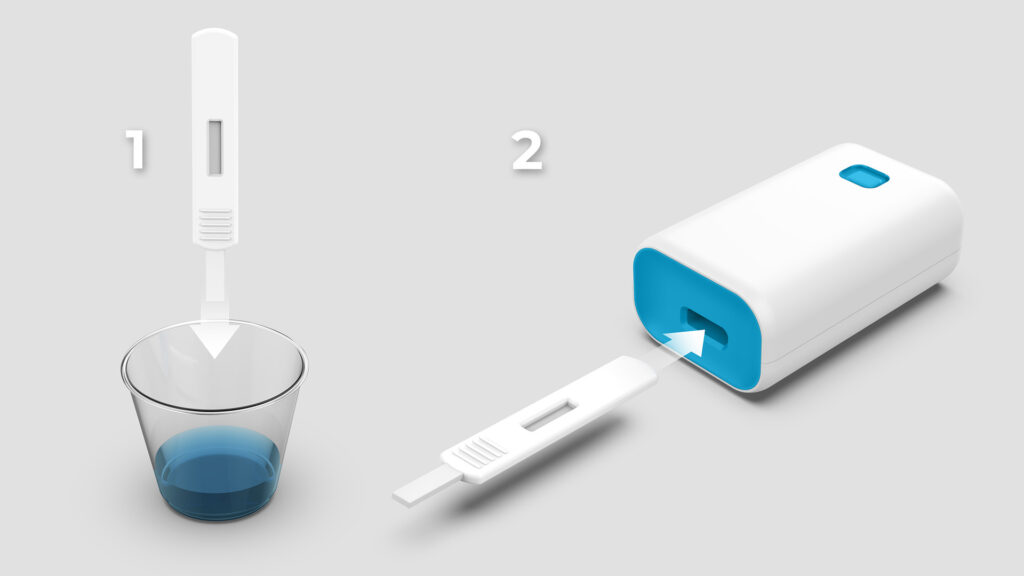
To address critical needs identified by the Biomedical Advanced Research and Development Authority (BARDA), Triple Ring was awarded a contract to design, build, and test a low-cost multiplexed quantitative biomarker detection platform for lab-at-home and low resource settings.
Outcomes
Triple Ring and a close collaborator, in a project funded by the Biomedical Advanced Research and Development Authority (BARDA), have developed a low-cost, user-centric, diagnostics platform for multiplexed quantitative biomarker measurements in patient samples. This novel platform was designed for use in CLIA-waived in vitro diagnostic settings and is notable for its low manufacturing cost. The platform supports lab-at-home, point of care (POC), and direct to consumer workflows especially in low-resource settings. Triple Ring’s consolidated expertise across biological, electrical, embedded software, mechanical, and optical systems enabled a robust design capable of quantifying biomarker concentrations from multiplexed lateral flow immunoassay (LFA) test strips. Additionally, the system was demonstrated to extend the quantitative measurement range beyond that of typical lateral flow assay readers. Currently, the team is performing systems integration and testing for a handheld version of the diagnostic device.
Triple Ring Value Proposition
- Multidisciplinary team including biochemistry, assay development, biology, computer science, optics, and multiple engineering disciplines to design, build, and test complex systems with very low manufacturing costs
- Focus on building transformational platforms and capabilities to target unmet needs in the in vitro diagnostics space, including development for low-resource and remote settings
- Valuable IP creation
- Company formation services through General Inception venture studio
At-Home Quantitative Diagnostics
Challenge
To address critical needs identified by the Biomedical Advanced Research and Development Authority (BARDA), Triple Ring was awarded a contract to design, build, and test a low-cost multiplexed quantitative biomarker detection platform for lab-at-home and low resource settings.
Outcomes
Triple Ring and a close collaborator, in a project funded by the Biomedical Advanced Research and Development Authority (BARDA), have developed a low-cost, user-centric, diagnostics platform for multiplexed quantitative biomarker measurements in patient samples. This novel platform was designed for use in CLIA-waived in vitro diagnostic settings and is notable for its low manufacturing cost. The platform supports lab-at-home, point of care (POC), and direct to consumer workflows especially in low-resource settings. Triple Ring’s consolidated expertise across biological, electrical, embedded software, mechanical, and optical systems enabled a robust design capable of quantifying biomarker concentrations from multiplexed lateral flow immunoassay (LFA) test strips. Additionally, the system was demonstrated to extend the quantitative measurement range beyond that of typical lateral flow assay readers. Currently, the team is performing systems integration and testing for a handheld version of the diagnostic device.
Triple Ring Value Proposition
- Multidisciplinary team including biochemistry, assay development, biology, computer science, optics, and multiple engineering disciplines to design, build, and test complex systems with very low manufacturing costs
- Focus on building transformational platforms and capabilities to target unmet needs in the in vitro diagnostics space, including development for low-resource and remote settings
- Valuable IP creation
- Company formation services through General Inception venture studio
Client
BARDA
Practice Areas

It’s very rewarding to work with our collaborators to develop new diagnostic tools to help protect the health of some of the most vulnerable populations around the world.
Microneedle Patch Applicator
Client
Confidential
Practice Areas
Challenge
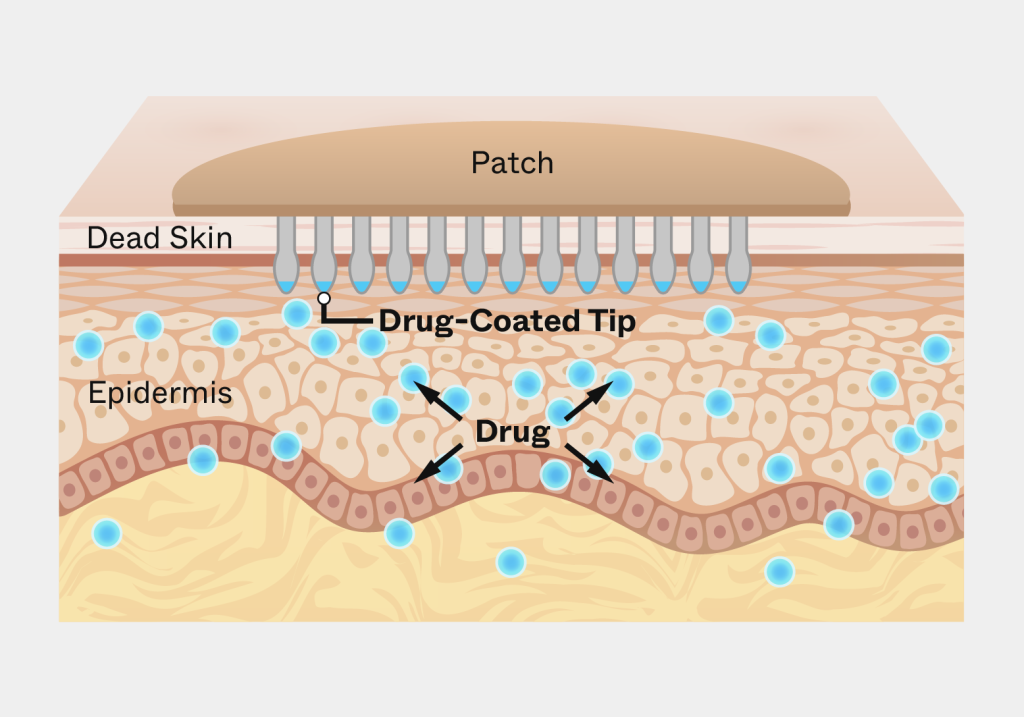
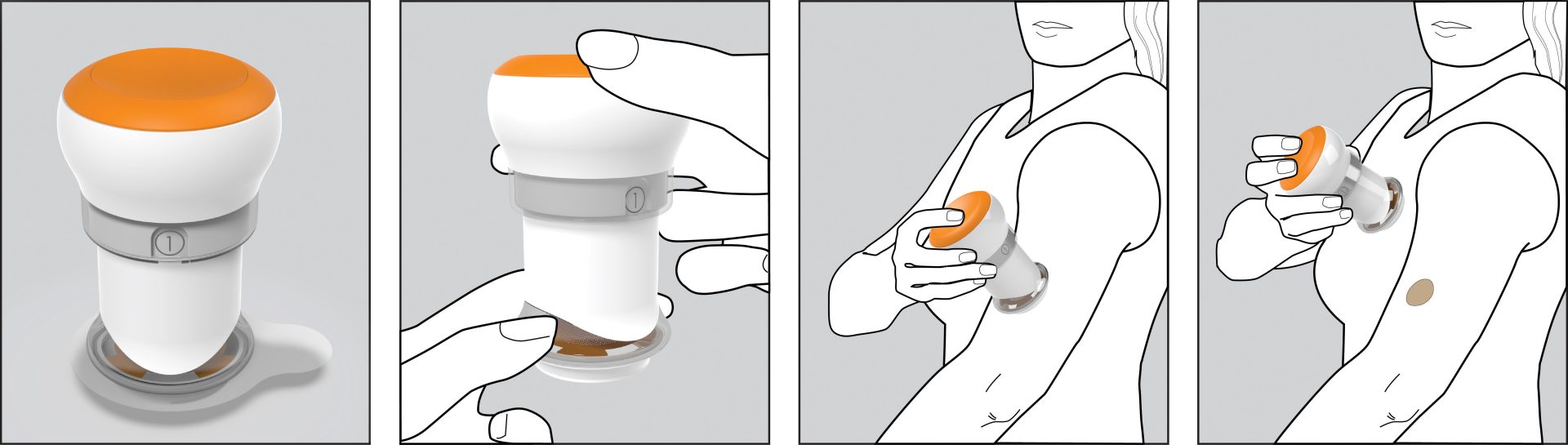
The client had developed a novel microneedle patch for transdermal drug delivery intended for use by the patient at home. The patch required a significantly greater degree of uniformity in pressure during application than existing transdermal patches. It required design of a reusable applicator to achieve reliable adhesion and dosing, while supporting the robustness and ruggedness requirements for use by a patient in their home and a low cost.
The key was ensuring that the patch would work across a wide variety of skin types in the potential patient population. Triple Ring relied on its expertise in material design and selection, precision mechanical engineering, and our life scientists to design an applicator that provided the required pressure for patch adhesion, across skin types that varied by thickness, age, moisture content, and placement.
Outcomes
The resulting single-entity combination product comprised a reusable applicator and a microneedle array containing patch, for intracutaneous drug delivery allowing rapid absorption into the bloodstream. With the applicator, the client was able to successfully enter clinical trials. Ultimately the technology for the product was acquired by a vaccine manufacturer.
Value Propositions
Triple Ring’s interdisciplinary team had all the required skills for a complex design.
Microneedle Patch Applicator
Challenge
The client had developed a novel microneedle patch for transdermal drug delivery intended for use by the patient at home. The patch required a significantly greater degree of uniformity in pressure during application than existing transdermal patches. It required design of a reusable applicator to achieve reliable adhesion and dosing, while supporting the robustness and ruggedness requirements for use by a patient in their home and a low cost.
The key was ensuring that the patch would work across a wide variety of skin types in the potential patient population. Triple Ring relied on its expertise in material design and selection, precision mechanical engineering, and our life scientists to design an applicator that provided the required pressure for patch adhesion, across skin types that varied by thickness, age, moisture content, and placement.
Outcomes
The resulting single-entity combination product comprised a reusable applicator and a microneedle array containing patch, for intracutaneous drug delivery allowing rapid absorption into the bloodstream. With the applicator, the client was able to successfully enter clinical trials. Ultimately the technology for the product was acquired by a vaccine manufacturer.
Value Propositions
Triple Ring’s interdisciplinary team had all the required skills for a complex design.
Client
Confidential
Practice Areas

Background
The client was a publicly traded company with deep medical and domain expertise to address a drug delivery problem using their novel patch technology. They selected Triple Ring to design the mechanical delivery device based upon our long history of working with designing novel technologies for health care that delivered excellent user experiences. The Triple Ring designed applicator allowed the client to both more easily demonstrate their technology to other health care professionals and to get it into the hands of end users in clinical trials.

IVD Platform Refresh
Client
Fortune 500 IVD Company
Practice Areas
Challenge
Our client faced the obsolescence of critical components within a legacy IVD product platform, including single-board computers and microcontrollers. A lack of original firmware source code and the sunsetting of institutional knowledge required extensive reverse engineering before updates could be designed and implemented. Adding further complexity, Triple Ring was tasked with modernizing the product to maintain market competitiveness yet demonstrate equivalence to fulfill the criteria of a Special 510(k) regulatory pathway.
Outcomes
A full system design and regulatory package was delivered for a refreshed IVD product with demonstrated equivalency to its FDA-cleared predecessor. Triple Ring replaced obsolete components, reverse-engineered and migrated existing software to a new OS, updated subassemblies, and developed new firmware compatible with legacy systems. The refreshed system featured modern industrial design, met manufacturing cost targets, and integrated seamlessly with existing manufacturing processes, ensuring uninterrupted production.
Value Propositions
- Multidisciplinary teams skilled in reverse engineering and future-proofing
- Software porting expertise
- In-depth knowledge of clinical IVD devices and relevant regulatory requirements
- Strong collaboration with client manufacturing and service teams
- Industrial Design capabilities to modernize user experience
IVD Platform Refresh
Challenge
Our client faced the obsolescence of critical components within a legacy IVD product platform, including single-board computers and microcontrollers. A lack of original firmware source code and the sunsetting of institutional knowledge required extensive reverse engineering before updates could be designed and implemented. Adding further complexity, Triple Ring was tasked with modernizing the product to maintain market competitiveness yet demonstrate equivalence to fulfill the criteria of a Special 510(k) regulatory pathway.
Outcomes
A full system design and regulatory package was delivered for a refreshed IVD product with demonstrated equivalency to its FDA-cleared predecessor. Triple Ring replaced obsolete components, reverse-engineered and migrated existing software to a new OS, updated subassemblies, and developed new firmware compatible with legacy systems. The refreshed system featured modern industrial design, met manufacturing cost targets, and integrated seamlessly with existing manufacturing processes, ensuring uninterrupted production.
Value Propositions
- Multidisciplinary teams skilled in reverse engineering and future-proofing
- Software porting expertise
- In-depth knowledge of clinical IVD devices and relevant regulatory requirements
- Strong collaboration with client manufacturing and service teams
- Industrial Design capabilities to modernize user experience
Client
Fortune 500 IVD Company
Practice Areas

Background
A large multinational corporation specializing in In Vitro Diagnostic (IVD) devices engaged with Triple Ring to refresh and future-proof a legacy product line facing component and software obsolescence. Triple Ring executed an accelerated program to modernize the platform while holding production costs steady and demonstrating equivalent performance.
Maximize Legacy Product Value
Client
Large Corporations
Practice Areas
Background
Sustaining engineering is more than product maintenance and implementing market requests; it’s a strategic approach to keeping legacy products competitive and profitable over decades. At Triple Ring, we don’t simply maintain legacy products – we transform them to meet modern demands. We add high-value features like AI in addition to updating industrial design, increasing reliability, and lowering production costs. We also address evolving regulatory risk, including cyber security, while carefully protecting core intellectual property.
Modernization of Legacy Products
Keeping products ahead in the market through:
- AI design and integration
- Cybersecurity software migrations
- Performance upgrades and added functionality
- Refreshed industrial design, usability, and aesthetics
- Supply chain security
Maximize Legacy Product Value
Background
Sustaining engineering is more than product maintenance and implementing market requests; it’s a strategic approach to keeping legacy products competitive and profitable over decades. At Triple Ring, we don’t simply maintain legacy products – we transform them to meet modern demands. We add high-value features like AI in addition to updating industrial design, increasing reliability, and lowering production costs. We also address evolving regulatory risk, including cyber security, while carefully protecting core intellectual property.
Modernization of Legacy Products
Keeping products ahead in the market through:
- AI design and integration
- Cybersecurity software migrations
- Performance upgrades and added functionality
- Refreshed industrial design, usability, and aesthetics
- Supply chain security
Client
Large Corporations
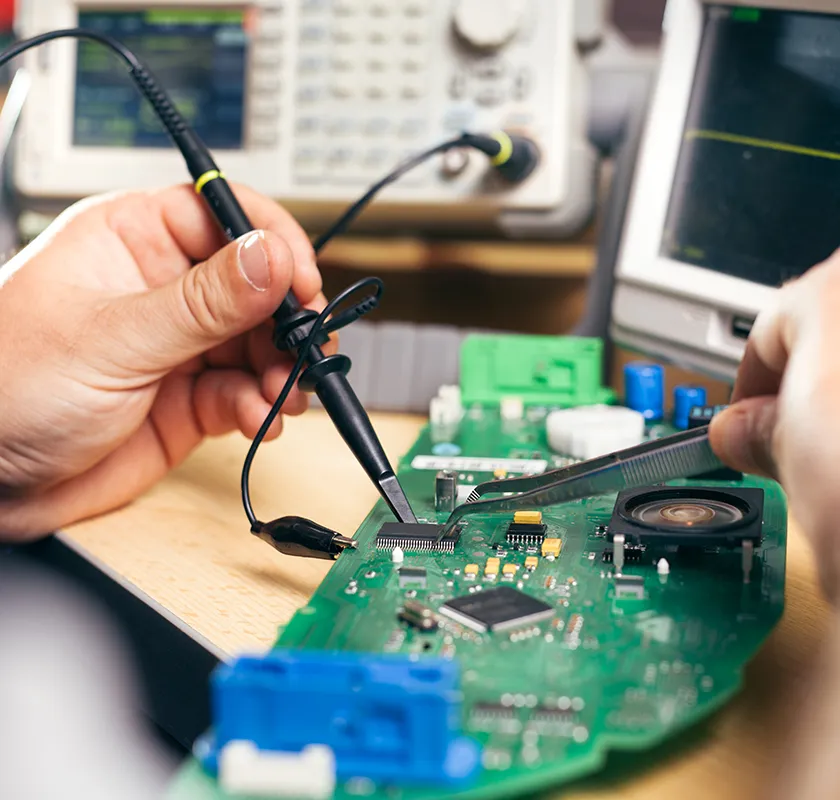

AI Enhancement
Enhancing legacy systems with physics-based AI improves performance, accuracy, and efficiency, and supports predictive maintenance. These features dramatically extend product lifespan without costly hardware upgrades. Triple Ring has the experience and expertise in AI algorithm design and integration to modernize products and create new valuable solutions for customers.
Supply Chain Optimization
Updating and optimizing supply chains addresses obsolescence, reduces cost, increases production yields and product reliability. Triple Ring and our partners ensure smooth production, minimize disruptions, and unlock new cost-saving opportunities for your legacy products.
Why Partner with Triple Ring?
Successful products often outlive the teams that launched them resulting in critical knowledge gaps within organizations that grow over time. Product companies need a trusted partner with deep expertise in reverse engineering, modern regulatory standards, supply chain access, and expertise in AI. Partnering with Triple ring unlocks these benefits, and importantly, saves product companies the cost and schedule delays associated with rebuilding engineering teams. Ensure product success and optimal return on investment over the long haul by engaging with Triple Ring on your next sustaining engineering project.