Triple Ring
Ensure success by partnering with Triple Ring’s dedicated teams of experts. Together we accelerate development of your breakthrough products.

Our mission is to keep you at the leading edge
At Triple Ring, we collaborate fearlessly to overcome the vexing challenges in medical technology development, including decreasing time to market, reducing cost, reducing technical risk, securing supply chains, and complying with regulatory requirements. Our cross-functional expertise and multidisciplinary teams in engineering, biology, and design work shoulder-to-shoulder with you to manage program risk and maintain tight control of schedule and budget.
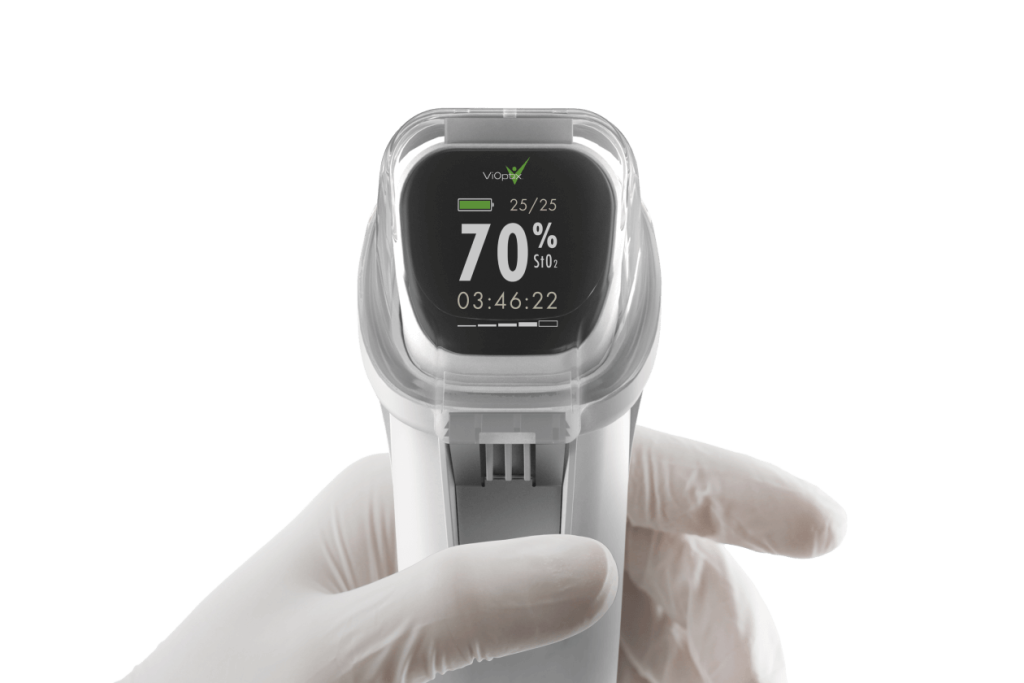
Triple Ring Accelerates ViOptix tissue oximeter to market
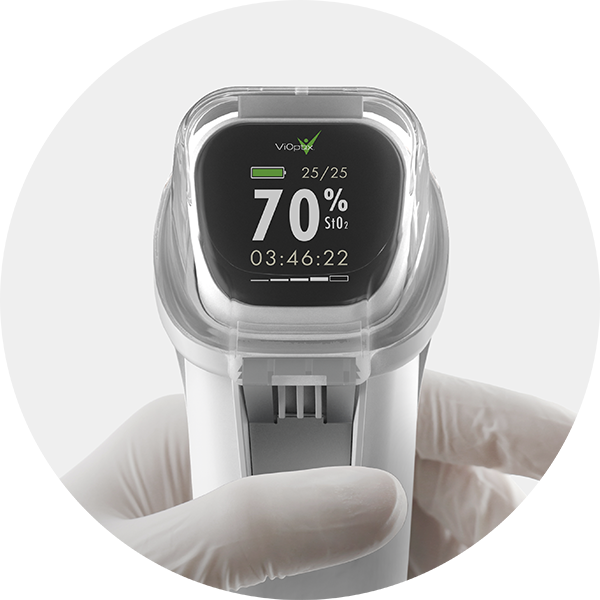
ViOptix partnered with Triple Ring to tackle complex development challenges, successfully launching the Intra.Ox multi-wavelength tissue oximeter in 2021.
Acknowledgments
I don’t think we could have found a higher caliber of scientists and engineers under one roof. They are flexible to work with and have grown with our needs. I would highly recommend them.
Partner with us to overcome difficult challenges
— Lack of dedicated, committed, and expert resources
— Reverse engineering and refresh of legacy products
— Keeping up with the latest advances, including AI
— Protecting market share and staying ahead of competition
— Incorporating AI into existing products
We strive to be a trusted innovation partner.
Working across multiple technology areas, Triple Ring helps you propel innovation at the forefront of medtech and life science: We let you empower clinicians with robotics, AI, and sensors for smarter, more cost-effective care. We translate your engineered-biology breakthroughs from lab-to-clinic. We ensure more sustainable and secure supply chains for healthcare.
Bridge the gap between ideas and commercial success
Collaborative innovation
We work closely with your team, combining our expertise with yours to tackle challenges, drive innovation, and create products that meet real-world needs.
Shared goals
Your success is our success. We align our efforts with your objectives and communicate thoughtfully, ensuring that every decision we make advances your product development goals.
Proactive problem-solving
We anticipate challenges before they arise, offering solutions that keep your project on track and ensuring a smoother path from concept to commercialization.


FAQ
We provide end-to-end services, from concept to market, including design, engineering, prototyping, and regulatory support. Our approach is tailored to your project’s unique needs.
Our team has deep experience navigating regulatory requirements. We ensure all products meet industry standards and work closely with regulatory bodies to streamline approvals. Our quality management system is certified to ISO 13485 and we are California FDB licensed for contract design, development, consulting, and manufacturing management of medical technologies. We develop software under and are certified to IEC 60601-1.
We take a knowledge-based approach to ensure we are identifying unknowns and adapting our methods to close those gaps. We follow a range of risk management strategies, including rigorous testing and iterative development, to identify and mitigate potential issues early in the process. Our team also ensures robust cybersecurity integration into medical device design QMS, safeguarding against emerging threats while meeting regulatory requirements.
Triple Ring stands out as a CDMO (contract development and manufacturing organization). Our team combines industry-leading expertise with a deep understanding of the relevant science, application domains, and the technologies required to deliver effective solutions.
It is possible. Together.
Developing new technology is inherently difficult and risky. As a co-development company, we strive to be a trusted partner, not simply a consultant. We structure projects to meet the business needs of our clients, and we often share risk.